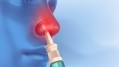
FDA approval of needle-free spray, a major breakthrough in anaphylaxis treatment
This marks the first significant innovation in epinephrine delivery in more than 35 years and offers an alternative to the anxiety-inducing needle injections that have long been the only emergency treatment available for anaphylaxis.
What is Anaphylaxis?
- Anaphylaxis is a severe, potentially life-threatening allergic reaction that can occur within minutes of exposure to an allergen.
- Common triggers include certain foods (e.g., peanuts, shellfish), medications, insect stings, and latex.
Symptoms
- Symptoms of anaphylaxis can vary but often include:
- Difficulty breathing or shortness of breath
- Swelling of the throat, tongue, or lips
- Rapid or weak pulse
- Skin reactions, including hives, itching, and flushing
- Nausea, vomiting, or diarrhea
- Dizziness or fainting
- A sense of impending doom
Prevalence
- An estimated 1 in 50 Americans experience anaphylaxis at some point in their lives.
- Anaphylaxis accounts for approximately 500,000 emergency room visits in the U.S. each year.
Treatment
- Immediate Administration of Epinephrine:
- Epinephrine is the first-line treatment for anaphylaxis and should be administered as soon as symptoms appear.
- The medication works by constricting blood vessels, relaxing muscles in the airways, and reducing swelling and hives.
- Traditional Delivery Method:
- Until recently, epinephrine has been administered via auto-injectors (e.g., EpiPen), which involve using a needle to inject the medication into the thigh.
Importance of Quick Response
- Delaying epinephrine administration can lead to worsening symptoms and increase the risk of a fatal outcome.
- Patients at risk of anaphylaxis are advised to carry an epinephrine device at all times and to use it immediately if they experience symptoms.
Epinephrine has been a lifesaver for patients experiencing type 1 allergic reactions, including those triggered by foods, medications, and insect stings. Traditionally, it has been administered through auto-injectors like EpiPen, which involve using a needle—a method that has proven effective but is often fraught with patient hesitancy due to needle phobia. In fact, studies show that many patients delay or even forgo administering the life-saving injection due to anxiety about using needles, which can lead to severe health outcomes.
“Until today, patients with severe allergic reactions only had one treatment option—an often painful and anxiety-inducing needle injection of epinephrine,” said Dr Thomas Casale, professor of medicine and pediatrics and chief of clinical and translational research at the University of South Florida’s Division of Allergy and Immunology.
“The FDA approval of neffy means that patients with severe allergies finally gain a long-awaited, needle-free, easy-to-carry epinephrine delivery method.”
The implications of this approval extend far beyond patient comfort. According to ARS Pharmaceuticals, an estimated 500,000 emergency room visits occur annually in the US due to anaphylaxis, with nearly 60% of these patients not having received epinephrine before arriving at the ER. The availability of a non-invasive, easy-to-use nasal spray could increase the timely administration of epinephrine, potentially reducing the severity of allergic reactions and improving patient outcomes.
Innovation without compromise
The FDA’s approval of neffy is supported by robust clinical data, including five primary registration studies that evaluated the safety and efficacy of the 2 mg intranasal dose. The results demonstrated that neffy’s pharmacokinetic (PK) and pharmacodynamic (PD) profiles are within the same range as those of existing epinephrine injection products. Clinical trials, which included healthy adults and pediatric patients weighing 30 kg (66 lbs.) or more, showed that adverse events were generally mild, with no significant nasal irritation or serious adverse events reported.
“We know that earlier administration of epinephrine is better, but for many, the needle is a barrier that causes dangerous hesitation,” said Dr Jonathan Spergel, chief of the allergy program at Children's Hospital of Philadelphia. “That is why the field has long pursued an effective treatment approach that does not require an injection.”
A new era for patients and caregivers
ARS Pharmaceuticals, the company behind neffy, has emphasized that the new delivery method is not only more convenient but also aligns with the needs and concerns of patients and caregivers. The ease of use and portability of neffy could encourage more consistent carrying and use of epinephrine among those at risk, potentially saving lives in emergency situations.
“This approval marks a watershed moment in addressing an unmet medical need for people with Type I allergies,” said Richard Lowenthal, co-founder, president, and CEO of ARS Pharmaceuticals.
“Our team has worked tirelessly to create an easy-to-carry, easy-to-use, needle-free device that offers peace of mind to patients and caregivers by enabling them to administer epinephrine quickly and confidently when needed.”
Expanding access and affordability
To ensure that neffy is accessible to all patients, ARS Pharmaceuticals has rolled out several patient support programs. For those with commercial insurance, the company is capping
out-of-pocket costs at $25 for a two-dose prescription via a co-pay savings program. Additionally, uninsured patients or those whose insurance does not cover neffy can purchase it for $199 through digital pharmacy services such as BlinkRx or GoodRx.
ARS Pharma is also offering a Patient Assistance Program (PAP) to provide neffy at no cost to eligible US residents who are uninsured or underinsured. The company says its commitment to affordability highlights the company’s mission to make this potentially life-saving treatment accessible to as many people as possible.
Looking ahead
neffy is expected to be available in the US within eight weeks of FDA approval for patients weighing 30 kg or more. ARS Pharma also plans to file for approval to extend neffy’s use to children weighing 15 to 30 kg by the end of the third quarter of 2024. The company is preparing for a commercial launch in the European Union by Q4 2024, following anticipated market authorization by the European Commission.
The approval of neffy represents a significant step forward in the treatment of severe allergic reactions, offering patients and caregivers a simpler, less intimidating option that could ultimately improve outcomes in emergencies. As Dr Lou Garrison, professor emeritus at the University of Washington, said: “Like a fire extinguisher in the home, neffy offers peace of mind to the person carrying it and also to their family and friends.”