Symbiosis expands sterile manufacturing with new facility and innovations

The event provides a great platform for the company to present its newest addition—a 20,000 sq ft facility in Stirling, UK, which the company says enhances Symbiosis' ability to support biopharma companies with high-quality manufacturing solutions. Colin MacKay, founder and CEO of Symbiosis, shared exclusive insights in a recent interview with Outsourcing Pharma.
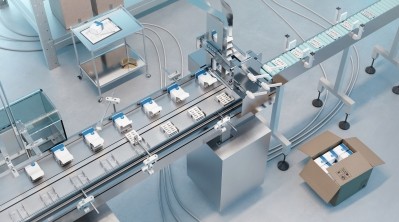
Symbiosis has long been known for its expertise in sterile fill/finish services, but the latest developments signal a significant step forward in its capabilities. "We're excited to announce our new facility, which features a $1.5 million fully automated fill/finish production line," MacKay said. This state-of-the-art equipment will play a critical role in the company's commercial supply of marketed medicines, providing much-needed capacity to meet growing demand. "The increased sterile manufacturing capabilities will allow us to offer more value to our clients, helping them meet clinical trial milestones and deliver life-saving medicines faster."
Goals for CPHI Milan
For MacKay, CPHI Milan is not just about showcasing new facilities; it's about highlighting how Symbiosis can help pharmaceutical companies navigate the challenges of drug development. "Our primary goal is to raise awareness with potential clients about how we can bring value to their drug development efforts," he explained. Symbiosis prides itself on offering accelerated manufacturing and batch release timelines, something that MacKay emphasizes as crucial in the competitive biopharma market.
"We enable our clients to get their products to market as quickly as possible while maintaining the highest GMP standards. That’s something we’re very proud of, and something we know our clients highly value."
Staying ahead of industry trends
The pharmaceutical landscape is constantly evolving, and staying ahead of trends is critical for any company. Symbiosis achieves this by maintaining close relationships with its clients and staying attuned to regulatory changes. "We routinely listen to our existing clients to gauge what’s trending and what CDMO service requirements may be emerging in the market," said MacKay. Additionally, Symbiosis’ quality team continuously monitors regulatory updates from agencies like the MHRA and FDA, ensuring compliance with the latest standards.
MacKay also highlights the importance of attending events like CPHI. "Conferences help us stay in touch with what's going on in the industry. They allow us to learn from peers and bring those insights back to our clients."
Overcoming industry challenges
While the post-pandemic era has eased many challenges for the pharmaceutical industry, MacKay acknowledges that supply chain risks and financing issues persist. "Supply chain risks remain due to broader political and environmental factors," he said. Additionally, smaller biotech companies have faced financing difficulties in recent years, although MacKay is optimistic about improvements in this area. "There are emerging signs of improvement in the financing market, and we're well-positioned to help our clients navigate these challenges."
Symbiosis has adopted a risk-mitigation strategy, including strong inventory management and business continuity plans, to ensure it remains resilient amid these challenges. This approach allows the company to continue growing while expanding its sterile manufacturing capabilities.
Plans for expansion
Looking ahead, Symbiosis has ambitious growth plans, starting with the new Stirling facility. The facility is expected to be fully qualified by early 2025 and will cater to the growing needs of biotechnology and pharmaceutical companies in both the US and Europe. MacKay shared that there is additional fallow space within the facility for future expansion, including plans for further automated filling line capacity.
"Symbiosis is poised for growth," MacKay said. "This new facility positions us perfectly to meet the increasing demands of our clients while maintaining the high standards that have made us a trusted partner in the industry."
GMP sterile fill/finish manufacturing capacity
One of the key elements that differentiate Symbiosis from its competitors, he tells us is its commitment to speed and flexibility. "Our clients tell us that they highly value our ability to offer GMP sterile fill/finish manufacturing capacity on a short lead time," said MacKay. Symbiosis focuses on saving time for its clients without compromising on compliance, allowing them to accelerate the release of their drug products—a critical factor in getting life-saving medicines to patients.
MacKay credits this success to the company’s culture and its dedicated team. "We've developed an operational strength over many years, driven by a team of excellent people. Our cultural approach emphasizes flexibility, speed, and absolute GMP compliance, and that’s what sets us apart."
With its new facility, expanded capabilities, and continued focus on innovation, Symbiosis says it is well-positioned to play a pivotal role in the future of sterile biopharmaceutical manufacturing.